From Polymers to Fine Chemicals: How Versatile Are High-Pressure Reactor Systems?
Struggling with low yields or slow reactions in your chemical processes? Wondering how to tackle challenging syntheses effectively? High-pressure reactors might be the breakthrough you need.
High-pressure reactor systems are incredibly versatile. They enable a wide range of chemical reactions, from polymer production to creating complex fine chemicals, by manipulating reaction conditions for better outcomes. This versatility makes them essential in many labs and industries.

As a manufacturer with over 16 years in this field, I've seen firsthand how essential the right equipment is. High Pressure Chemical Reactors are fascinating. They are not just containers; they are environments we create to persuade molecules to do extraordinary things. This ability to control pressure opens up a whole new world for chemists and material scientists. It allows us to push boundaries and explore new chemical pathways that are simply not possible at normal atmospheric pressure. Let's explore why these systems are such a game-changer for so many applications.
How Do High-Pressure Reactors Revolutionize Polymer Synthesis?
Finding it hard to control polymer chain length or dealing with stubborn monomers? Traditional methods can be limiting. High-pressure reactors offer precise control for superior polymer products.
High-pressure reactors revolutionize polymer synthesis by enabling reactions with gaseous monomers, increasing reaction rates, and allowing fine control over molecular weight and polymer architecture. This leads to higher quality and more specialized polymers.
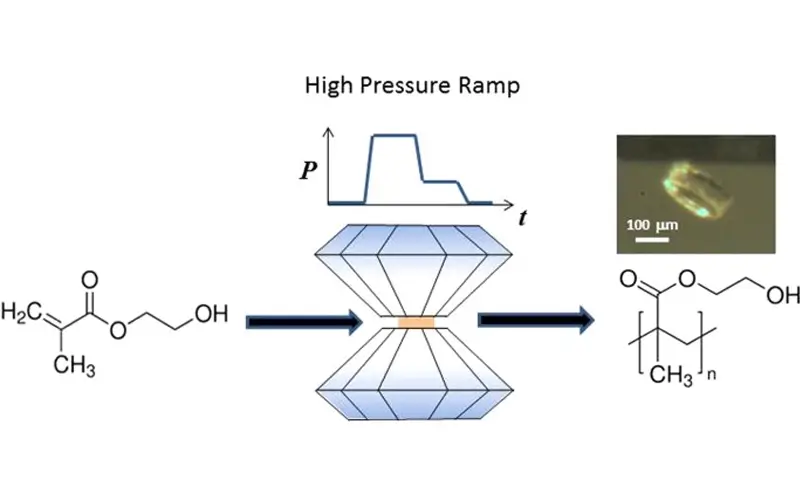
When we talk about making polymers, control is key. High pressure gives us that control. Think about monomers that are gases at room temperature, like ethylene. How do you get them to react efficiently? You increase the pressure. This forces more gas molecules into the liquid phase where the reaction happens. This means faster reactions and better incorporation of the monomer into the polymer chain.
I remember a client who was struggling to produce a specific copolymer with consistent properties. Their existing setup just couldn't handle the volatile monomer effectively. Once they switched to one of our high-pressure reactor systems, they saw a dramatic improvement in both yield and product consistency. It was a real turning point for their research.
Key Advantages in Polymerization:
Enhanced Reaction Rates: Higher pressure increases reactant concentration, speeding up polymerization.
Improved Monomer Solubility: Essential for gaseous monomers like ethylene or propylene.
Control Over Molecular Weight: Pressure can influence chain initiation, propagation, and termination steps.
Tailoring Polymer Properties: Allows for the creation of polymers with specific densities or crystallinities.
After the polymerization reaction in a high-pressure reactor, the resulting polymer often needs to be separated from unreacted monomers or solvents. This is where efficient downstream processing becomes vital. For example, if the polymer is in a solution, a rotary evaporator can be very useful for carefully removing the solvent without degrading the polymer, helping to isolate the pure product. We've designed our rotary evaporators to handle a range of solvents and volumes, making them a great partner to our reactor systems for a complete workflow.
Can High-Pressure Systems Enhance Fine Chemical Production?
Are your fine chemical syntheses suffering from low selectivity or poor yields? Complex molecules often demand more than standard conditions. High pressure can be the missing piece.
Yes, high-pressure systems significantly enhance fine chemical production by improving reaction selectivity, increasing yields, and enabling reactions that are otherwise difficult or impossible at atmospheric pressure. They are vital for creating complex, high-value compounds.

Fine chemicals are all about precision. These are often complex molecules used in pharmaceuticals, agrochemicals, or specialty materials. The synthesis routes can be long and challenging. High pressure can make a big difference here. It can shift the equilibrium of a reaction to favor the desired product, or it can increase the rate of a slow step. This means you get more of what you want and less of what you don't.
One common application is in hydrogenations, which are crucial for making many fine chemicals. Dissolving enough hydrogen gas into the reaction mixture is key, and high pressure makes this much easier and faster. I recall a project where a pharmaceutical intermediate required a very specific hydrogenation step. At atmospheric pressure, the reaction was sluggish and produced many side products. Using a high-pressure reactor not only sped up the reaction but also dramatically improved the purity of the product. This saved them a lot of time and resources in downstream purification.
Impact on Fine Chemical Synthesis:
Increased Selectivity: Pressure can influence the reaction pathway, favoring specific isomers or products.
Higher Yields: By overcoming activation energy barriers or shifting equilibria.
Access to Novel Chemistry: Enabling reactions like carbonylations or hydroformylations effectively.
Greener Processes: Sometimes, high pressure allows for reactions in more environmentally friendly solvents or even solvent-free conditions.
Once the synthesis in the high-pressure reactor is complete, isolating the target fine chemical is crucial. Often, these valuable products are present in a solvent mixture. This is another area where our rotary evaporators shine. They provide a gentle and efficient way to remove solvents, concentrating the fine chemical without decomposing it. Many of our clients in the pharmaceutical and specialty chemical sectors pair our high-pressure reactors with our rotary evaporators to create a seamless process from synthesis to initial product isolation. This careful handling is essential when dealing with sensitive, high-value compounds.
What Role Does Pressure Play in Catalytic Reactions and Hydrogenation?
Facing challenges with catalyst activity or gas solubility in your reactions? Many catalytic processes, especially hydrogenations, underperform at standard pressures. High pressure can unlock their full potential.
Pressure plays a critical role by increasing gas solubility (e.g., hydrogen, CO), enhancing catalyst-reactant interaction, and improving reaction rates and selectivity in many catalytic processes. This is especially true for hydrogenation and carbonylation reactions.

Catalysts are amazing – they speed up reactions without being consumed. But sometimes, they need a little help. That's where high pressure comes in, especially when gases are involved, like in hydrogenation (using hydrogen gas) or carbonylation (using carbon monoxide). The main job of pressure here is to get more gas molecules to dissolve in the liquid where the reaction is happening. More dissolved gas means more chances for the gas to interact with the catalyst and the other reactants.
Think of it like trying to dissolve sugar in iced tea versus hot tea. It's easier in hot tea. Pressure acts similarly for gases in liquids. For hydrogenation, this is vital. We want the hydrogen to find the catalyst and the molecule we are trying to hydrogenate. High pressure ensures there's plenty of hydrogen available right where it's needed. We've worked with many research institutions that use our high-pressure reactors specifically for developing new catalysts and optimizing hydrogenation processes for things like converting oils into fats or producing pharmaceutical ingredients.
Benefits for Catalytic Reactions:
| Aspect | Benefit of High Pressure |
|---|---|
| Gas Solubility | Significantly increases the concentration of gaseous reactants (H₂, CO, O₂) in the liquid phase. |
| Reaction Rate | Often accelerates the reaction due to higher reactant concentrations. |
| Catalyst Stability/Activity | Can sometimes improve catalyst lifetime or activity by maintaining a certain phase or preventing side reactions. |
| Selectivity | Can influence the reaction pathway, leading to a higher proportion of the desired product. |
After a successful catalytic reaction under high pressure, the product needs to be separated from the catalyst (if it's a heterogeneous catalyst) and the solvent. Filtration might remove the catalyst, but then you are often left with the product dissolved in a solvent. This is where careful solvent removal is important. Our rotary evaporators are ideal for this step, gently concentrating the product. For example, after a hydrogenation to create a specific organic compound, the rotary evaporator can efficiently strip away the reaction solvent, leaving behind the purified hydrogenated product. This integration of reactor and evaporator technology streamlines the workflow for many of our customers.
Beyond Synthesis: How Are High-Pressure Reactors Used in Material Science?
Are you exploring new materials with unique properties but finding conventional methods insufficient? Material science often requires extreme conditions. High-pressure reactors offer a pathway to innovation.
In material science, high-pressure reactors are used for synthesizing novel materials like advanced ceramics, alloys, and nanomaterials through processes such as hydrothermal synthesis, solvothermal synthesis, and creating materials stable only under high pressure.

Material science is all about creating new stuff with amazing properties – super strong, super light, or with unique electronic capabilities. High-pressure reactors are like a special tool for material scientists. They allow researchers to force atoms and molecules together in ways that wouldn't happen normally. This can lead to completely new materials or existing materials with improved characteristics.
One common technique is hydrothermal synthesis. Here, you use water as a solvent, but at high pressure and temperature, water behaves very differently. It can dissolve things it normally wouldn't, helping to create fine crystals of materials like zeolites (used as catalysts or filters) or certain types of ceramics. I remember a university lab that acquired one of our high-pressure systems to explore new semiconductor nanocrystals. The precise control over temperature and pressure was critical for them to grow crystals of the exact size and quality they needed for their experiments in next-generation electronics.
Applications in Material Science:
Hydrothermal/Solvothermal Synthesis: Creating crystalline materials like zeolites, metal-organic frameworks (MOFs), and nanoparticles.
Phase Transformation Studies: Investigating how materials change structure under extreme pressure.
Synthesis of Superhard Materials: Creating materials like synthetic diamonds or cubic boron nitride.
Material Densification: Compacting powdered materials to form dense solids.
After synthesizing these novel materials, especially if they are in a solution or suspension, researchers need to isolate them. If solvents other than water are used in solvothermal processes, or if washing steps involve organic solvents, these solvents need to be removed. While some materials are simply filtered and dried in an oven, for sensitive nanomaterials or when precise solvent removal is needed to avoid agglomeration, a rotary evaporator can be a valuable tool. It allows for controlled evaporation at lower temperatures, which can be crucial for preserving the structure and properties of delicate, newly synthesized materials. We ensure our equipment supports the innovative work happening at the frontiers of science.
Ensuring Safety and Efficiency: What Key Features Should You Look for in High-Pressure Reactors?
Worried about the risks of high-pressure experiments or inefficient lab workflows? Choosing the right reactor with proper features is crucial. Safety and efficiency should be top priorities.
Key features include robust construction materials, precise pressure and temperature controls, reliable safety mechanisms (like rupture discs and relief valves), and user-friendly design for efficient operation and maintenance. These ensure both safety and reliable results.

Working with high pressures isn't something to take lightly. Safety is absolutely paramount. As a manufacturer, this is our top concern. The reactor itself must be built from materials that can withstand the pressure and the chemicals you're using – often stainless steel or specialized alloys like Hastelloy. Then, there are the safety mechanisms. A rupture disc is a must-have; it's designed to burst at a set pressure to prevent a catastrophic failure. Pressure relief valves are also critical, as are accurate pressure gauges and temperature sensors.
I always tell my clients, "Your safety systems are your best friends when working under pressure." We once had a customer who was new to high-pressure work. We spent extra time training their team not just on operating the equipment, but on understanding all the safety interlocks and emergency procedures. That peace of mind is invaluable.
Critical Features for Safety and Efficiency:
Material of Construction: Must be compatible with reactants and conditions (e.g., SS316L, Hastelloy).
Pressure Control: Accurate pressure regulators, transducers, and gauges.
Temperature Control: Reliable heating/cooling systems with precise sensors.
Safety Interlocks: Over-pressure protection (rupture disc, relief valve), over-temperature cut-offs.
Agitation System: Efficient stirring for homogenous reaction conditions (e.g., magnetic drive).
Ease of Use & Maintenance: Clear controls, easy assembly/disassembly for cleaning.
Efficiency in the lab isn't just about the reaction time; it's about the entire workflow. After your high-pressure reaction is complete, how quickly and effectively can you move to the next step? This is where integrated solutions come into play. For instance, if your product needs to be concentrated or a solvent needs to be removed, having a reliable rotary evaporator ready can save significant time. Our philosophy is to provide not just standalone instruments, but solutions that work together. A well-designed high-pressure reactor ensures your synthesis is successful and safe, and efficient downstream equipment like our rotary evaporators helps you get to your final product faster. This combination boosts overall laboratory productivity.
As someone who has been in the lab instrument business for many years, I've learned that good equipment isn't just about features; it's about reliability and the support that comes with it. When you choose a reactor, you're also choosing a partner in your research or production.
Conclusion
High-pressure reactors are truly versatile tools, vital for innovation in chemistry and material science. They enable better reactions, leading to new products and discoveries across many fields.
