Stainless Steel Reactors in Pharmaceutical Manufacturing: A Comprehensive Guide
Introduction to Stainless Steel Reactors in Pharma
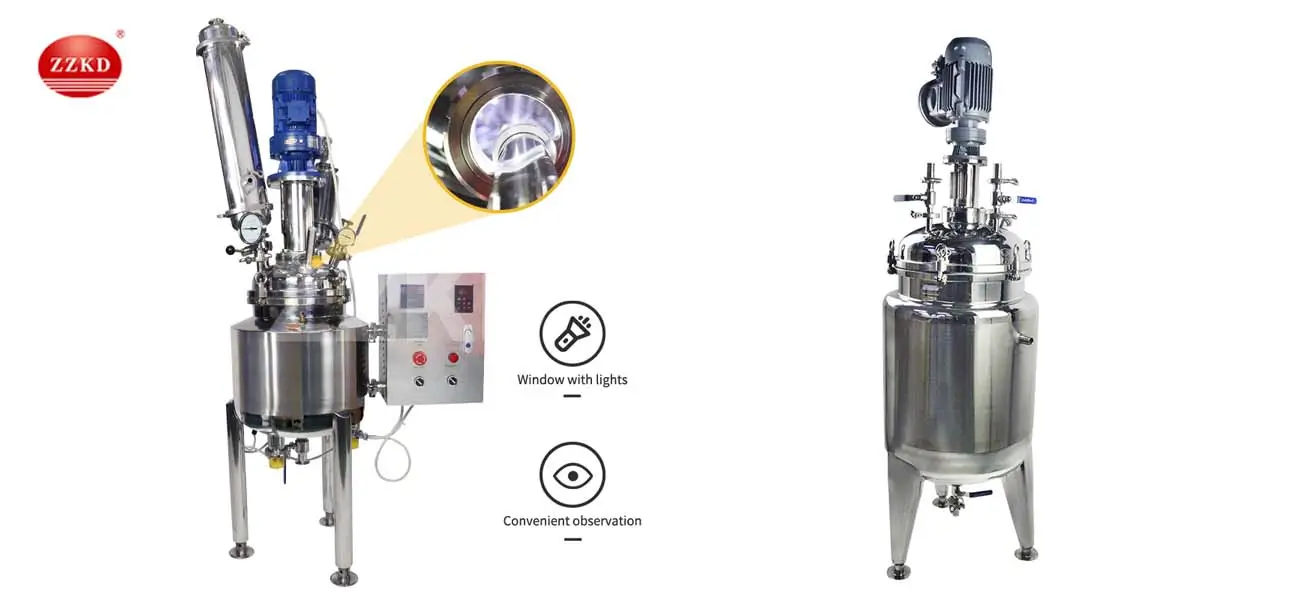
The pharmaceutical industry demands precision, hygiene, and regulatory compliance at every production stage. Stainless steel reactors have emerged as the backbone of modern drug manufacturing due to their corrosion resistance, thermal stability, and adherence to Good Manufacturing Practices (GMP). This article explores how these reactors enable critical processes like active pharmaceutical ingredient (API) synthesis, crystallization, and sterilization while maintaining stringent quality control.
Key Pharmaceutical Applications of Stainless Steel Reactors
1. API Synthesis: Chemical Precision Under Controlled Conditions
Active Pharmaceutical Ingredients (APIs) require exact temperature, pressure, and mixing parameters. Stainless steel reactors excel in:
Multi-step synthesis: Acid-catalyzed reactions (e.g., aspirin production)
Hydrogenation: Controlled H₂ gas reactions for antibiotic intermediates
Polymerization: Vaccine adjuvant development (e.g., polysaccharide conjugation)
Case Example: A European pharma company reduced API batch rejection rates by 40% after switching to 316L stainless steel jacketed reactors with automated PID temperature control.

2. Crystallization: Achieving Purity Through Material Superiority
Crystal size distribution directly impacts drug bioavailability. Stainless steel reactors enable:
Anti-solvent addition: Ethanol/water systems for penicillin crystallization
Cooling crystallization: Paracetamol production with ±0.5°C accuracy
Seeding control: Dedicated ports for seed crystal introduction
Technical Advantage: Electropolished interior surfaces (Ra <0.5 μm) prevent unwanted nucleation sites.

3. Sterilization & Cleaning: Meeting 21 CFR Part 11 Compliance
Pharma-grade reactors integrate:
CIP/SIP systems: Automated Clean-in-Place/Steam-in-Place cycles
Drainage design: 3° slope towards bottom valve (zero liquid retention)
Surface finish: Mirror polishing for bacterial growth prevention
Regulatory Note: ASME BPE-2019 compliant reactors reduce FDA audit findings related to cross-contamination.
Technical Specifications Driving Pharma Adoption
| Parameter | Pharmaceutical Requirement | Standard Reactor Features |
| Material Grade | 316L or higher | 316Ti with 2.1% molybdenum |
| Pressure Range | -0.1 to 6 bar | Full vacuum to 10 bar |
| Temperature Control | -20°C to 200°C | Double jackets for cryo/heating |
| Mixing Efficiency | 95% homogeneity in <5 mins | 3-blade pitched turbine impellers |
5 Reasons Pharma Companies Choose Stainless Steel Over Glass-Lined Reactors
Durability: Withstands 500+ CIP cycles vs. glass-lined reactors' 200-cycle limit
Thermal Shock Resistance: Handles rapid ΔT up to 150°C/min
Cleaning Validation: Meets USP <1072> guidelines for residue limits
Scalability: 50L to 20,000L capacities with identical surface finish
Lifecycle Cost: 30% lower TCO over 10 years compared to glass-lined alternatives
Maintenance Best Practices for Pharma Reactors
Passivation Schedule: 15% nitric acid treatment every 6 months
Gasket Management: Replace PTFE seals after 50 sterilization cycles
Agitator Alignment: Laser alignment checks prevent shaft wobble (max 0.05mm runout)

Future Trends: Smart Reactors in Pharma 4.0
Emerging integrations include:
PAT (Process Analytical Technology) ports for real-time HPLC monitoring
IIoT-enabled predictive maintenance using vibration/temperature sensors
Blockchain-compliant data logging for supply chain transparency
Conclusion: Optimizing Pharma Production with Advanced Reactor Technology
From small-molecule APIs to monoclonal antibodies, stainless steel reactors provide the material science foundation for safe, efficient pharmaceutical manufacturing. Their compatibility with automation, regulatory requirements, and high-value compounds makes them indispensable in modern pharma facilities.
ZZKD delivers GMP-ready stainless steel reactors with:
✅ ASME Section VIII Division 1 certification
✅ 3D factory acceptance testing reports
✅ IQ/OQ/PQ documentation support
Contact our engineers to design your pharmaceutical-grade reactor system today.
